When traditional conservation fails, science is using “assisted evolution” to give vulnerable wildlife a chance.
For tens of millions of years, Australia has been a playground for evolution, and the land Down Under lays claim to some of the most remarkable creatures on Earth.
It is the birthplace of songbirds, the land of egg-laying mammals and the world capital of pouch-bearing marsupials, a group that encompasses far more than just koalas and kangaroos. (Behold the bilby and the bettong!) Nearly half of the continent’s birds and roughly 90 per cent of its mammals, reptiles and frogs are found nowhere else on the planet.
Australia has also become a case study in what happens when people push biodiversity to the brink. Habitat degradation, invasive species, infectious diseases and climate change have put many native animals in jeopardy and given Australia one of the worst rates of species loss in the world.
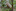
In some cases, scientists say, the threats are so intractable that the only way to protect Australia’s unique animals is to change them. Using a variety of techniques, including crossbreeding and gene editing, scientists are altering the genomes of vulnerable animals, hoping to arm them with the traits they need to survive.
“We’re looking at how we can assist evolution,” said Anthony Waddle, a conservation biologist at Macquarie University in Sydney.
It is an audacious concept, one that challenges a fundamental conservation impulse to preserve wild creatures as they are. But in this human-dominated age — in which Australia is simply at the leading edge of a global biodiversity crisis — the traditional conservation playbook may no longer be enough, some scientists said.
“We’re searching for solutions in an altered world,” said Dan Harley, a senior ecologist at Zoos Victoria. “We need to take risks. We need to be bolder.”
The extinction vortex
The helmeted honeyeater is a bird that demands to be noticed, with a patch of electric-yellow feathers on its forehead and a habit of squawking loudly as it zips through the dense swamp forests of the state of Victoria. But over the last few centuries, humans and wildfires damaged or destroyed these forests, and by 1989, just 50 helmeted honeyeaters remained, clinging to a tiny sliver of swamp at the Yellingbo Nature Conservation Reserve.
Intensive local conservation efforts, including a captive breeding programme at Healesville Sanctuary, a Zoos Victoria park, helped the birds hang on. But there was very little genetic diversity among the remaining birds — a problem common in endangered animal populations — and breeding inevitably meant inbreeding. “They have very few options for making good mating decisions,” said Paul Sunnucks, a wildlife geneticist at Monash University in Melbourne.
In any small, closed breeding pool, harmful genetic mutations can build up over time, damaging animals’ health and reproductive success, and inbreeding exacerbates the problem. The helmeted honeyeater was an especially extreme case. The most inbred birds left one-tenth as many offspring as the least inbred ones, and the females had life spans that were half as long, Sunnucks and his colleagues found.
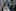
Without some kind of intervention, the helmeted honeyeater could be pulled into an “extinction vortex,” said Alexandra Pavlova, an evolutionary ecologist at Monash. “It became clear that something new needs to be done.”
A decade ago, Pavlova, Sunnucks and several other experts suggested an intervention known as genetic rescue, proposing to add some Gippsland yellow-tufted honeyeaters and their fresh DNA to the breeding pool.
The helmeted and Gippsland honeyeaters are members of the same species, but they are genetically distinct subspecies that have been evolving away from each another for roughly the last 56,000 years. The Gippsland birds live in drier, more open forests and are missing the pronounced feather crown that give helmeted honeyeaters their name.
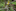
Genetic rescue was not a novel idea. In one widely cited success, scientists revived the tiny, inbred panther population of Florida by importing wild panthers from a separate population from Texas.
But the approach violates the traditional conservation tenet that unique biological populations are sacrosanct, to be kept separate and genetically pure. “It really is a paradigm shift,” said Sarah Fitzpatrick, an evolutionary ecologist at Michigan State University who found that genetic rescue is underused in the United States.
Crossing the two types of honeyeaters risked muddying what made each subspecies unique and creating hybrids that were not well suited for either niche. Moving animals between populations can also spread disease, create new invasive populations or destabilize ecosystems in unpredictable ways.
Genetic rescue is also a form of active human meddling that violates what some scholars refer to as conservation’s “ethos of restraint” and has sometimes been critiqued as a form of playing God.
“There was a lot of angst among government agencies around doing it,” said Andrew Weeks, an ecological geneticist at the University of Melbourne who began a genetic rescue of the endangered mountain pygmy opossum in 2010. “It was only really the idea that the population was about to go extinct that I guess gave government agencies the nudge.”
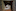
Sunnucks and his colleagues made the same calculation, arguing that the risks associated with genetic rescue were small — before the birds’ habitats were carved up and degraded, the two subspecies did occasionally interbreed in the wild — and paled in comparison with the risks of doing nothing.
And so, since 2017, Gippsland birds have been part of the helmeted honeyeater breeding programme at Healesville Sanctuary. In captivity there have been real benefits, with many mixed pairs producing more independent chicks per nest than pairs composed of two helmeted honeyeaters. Dozens of hybrid honeyeaters have now been released into the wild. They seem to be faring well, but it is too soon to say whether they have a fitness advantage.
Monash and Zoos Victoria experts are also working on the genetic rescue of other species, including the critically endangered Leadbeater’s opossum, a tiny, tree-dwelling marsupial known as the forest fairy. The lowland population of the opossum shares the Yellingbo swamps with the helmeted honeyeater; in 2023, just 34 lowland opossums remained. The first genetic rescue joey was born at Healesville Sanctuary last month.
The scientists hope that boosting genetic diversity will make these populations more resilient in the face of whatever unknown dangers might arise, increasing the odds that some individuals possess the traits needed to survive. “Genetic diversity is your blueprint for how you contend with the future,” Harley of Zoos Victoria said.
Targeting threats
For the northern quoll, a small marsupial predator, the existential threat arrived nearly a century ago, when the invasive, poisonous cane toad landed in eastern Australia. Since then, the toxic toads have marched steadily westward — and wiped out entire populations of quolls, which eat the alien amphibians.
But some of the surviving quoll populations in eastern Australia seem to have evolved a distaste for toads. When scientists crossed toad-averse quolls with toad-naive quolls, the hybrid offspring also turned up their tiny pink noses at the toxic amphibians.
What if scientists moved some toad-avoidant quolls to the west, allowing them to spread their discriminating genes before the cane toads arrived? “You’re essentially using natural selection and evolution to achieve your goals, which means that the problem gets solved quite thoroughly and permanently,” said Ben Phillips, a population biologist at Curtin University in Perth who led the research.

A field test, however, demonstrated how unpredictable nature can be. In 2017, Phillips and his colleagues released a mixed population of northern quolls on a tiny, toad-infested island. Some quolls did interbreed, and there was preliminary evidence of natural selection for “toad-smart” genes.
But the population was not yet fully adapted to toads, and some quolls ate the amphibians and died, Phillips said. A large wildfire also broke out on the island. Then, a cyclone hit. “All of these things conspired to send our experimental population extinct,” Phillips said. The scientists did not have enough funding to try again, but “all the science lined up,” he added.
Advancing science could make future efforts even more targeted. In 2015, for instance, scientists created more heat-resistant coral by crossbreeding colonies from different latitudes. In a proof-of-concept study from 2020, researchers used the gene-editing tool known as CRISPR to directly alter a gene involved in heat tolerance.

CRISPR will not be a practical, real-world solution anytime soon, said Line Bay, a biologist at the Australian Institute of Marine Science who was an author of both studies. “Understanding the benefits and risks is really complex,” she said. “And this idea of meddling with nature is quite confronting to people.”
But there is growing interest in the biotechnological approach. Waddle hopes to use the tools of synthetic biology, including CRISPR, to engineer frogs that are resistant to the chytrid fungus, which causes a fatal disease that has already contributed to the extinction of at least 90 amphibian species.
The fungus is so difficult to eradicate that some vulnerable species can no longer live in the wild. “So either they live in glass boxes forever,” Waddle said, “or we come up with solutions where we can get them back in nature and thriving.”
Unintended consequences
Still, no matter how sophisticated the technology becomes, organisms and ecosystems will remain complex. Genetic interventions are “likely to have some unintended impacts,” said Tiffany Kosch, a conservation geneticist at the University of Melbourne who is also hoping to create chytrid-resistant frogs. A genetic variant that helps frogs survive chytrid might make them more susceptible to another health problem, she said.
There are plenty of cautionary tales, efforts to reengineer nature that have backfired spectacularly. The toxic cane toads, in fact, were set loose in Australia deliberately, in what would turn out to be a deeply misguided attempt to control pest beetles.
But some environmental groups and experts are uneasy about genetic approaches for other reasons, too. “Focusing on intensive intervention in specific species can be a distraction,” said Cam Walker, a spokesperson for Friends of the Earth Australia. Staving off the extinction crisis will require broader, landscape-level solutions such as halting habitat loss, he said.
Moreover, animals are autonomous beings, and any intervention into their lives or genomes must have “a very strong ethical and moral justification” — a bar that even many traditional conservation projects do not clear, said Adam Cardilini, an environmental scientist at Deakin University in Victoria.
Chris Lean, a philosopher of biology at Macquarie University, said he believed in the fundamental conservation goal of “preserving the world as it is for its heritage value, for its ability to tell the story of life on Earth.” Still, he said he supported the cautious, limited use of new genomic tools, which may require us to reconsider some long-standing environmental values.
In some ways, assisted evolution is an argument — or, perhaps, an acknowledgment — that there is no stepping back, no future in which humans do not profoundly shape the lives and fates of wild creatures.
To Harley, it has become clear that preventing more extinctions will require human intervention, innovation and effort. “Let’s lean into that, not be daunted by it,” he said. “My view is that 50 years from now, biologists and wildlife managers will look back at us and say, ‘Why didn’t they take the steps and the opportunities when they had the chance?’”
This article originally appeared in The New York Times.
Written by: Emily Anthes
Photographs by: Chang W. Lee
©2024 THE NEW YORK TIMES
