New Zealand's medicines agency has become a lightning rod for controversy as it juggles public demands for life-saving medicines and a tight government budget. Matt Nippert digs into the arguments and asks: is it sustainable?
Julie Warwick was at home in Lyttleton in 1995 when the phone rang. It was her GP.
"I've got some bad news," the doctor said. She had hepatitis C.
The prognosis was stark. Hepatitis C, a blood-borne virus that affects about 50,000 New Zealanders, damages the liver and eventually leads to cirrhosis and early death. Warwick was already halfway there.
Warwick, now 65, has been a nurse all her working life, so she'd seen the suffering hepatitis C patients endure in the terminal stage of liver failure — their eyes and skin yellowed, confusion setting in as their brains clouded with toxins.
"It's not a nice way to go," she said.
But then came a series of medical advances that saved Warwick's life.
In the 2000s, scientists developed a drug called sofosbuvir that could cure hepatitis C quickly without the side effects of existing treatments. In 2016, Warwick got hold of the drug through an Australian doctor and immediately noticed improvements.
Six years later, she is cured of the disease, and she said her life has "completely changed".
"I've made decisions about doing things I wouldn't have done before. I've built myself a house. I'm back studying. And I have goals in my life again."
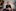
Today, Warwick works with hepatitis C patients at a clinic in Christchurch, who face a much better outlook than she did when she was diagnosed. In the past five years, New Zealand's medicines-buying agency, Pharmac, approved a new generation of hepatitis C treatments similar to those that saved Warwick's life.
As a result, thousands of Kiwis now have access to a simple and highly effective treatment, the cost of which is covered by the Government.
This is a story you don't often read about Pharmac. Media coverage of the agency typically focuses on decisions to deny funding for certain medicines and the anguish of desperate Kiwis whose lives depend on getting those drugs. The agency has become a lightning rod for controversy as it struggles to meet public expectations for medical treatment in a world of sharply rising drug prices and strained government budgets.
Campaigners and politicians have called for more funding and sweeping reform. Earlier this year, the Ardern government launched a formal review of the agency. Questions about its future will be back on the table again next month when the reviewers publish their interim recommendations.
Ahead of that review, the Herald spoke to patients, clinicians, industry analysts, drug company representatives and ministers, and reviewed hundreds of pages of internal agency documents, to understand how well Pharmac is working — and whether it is still fit for purpose.
Founded in 1993, Pharmac's mission is to "choose medicines to fund that will get the best health outcomes for New Zealanders". It is a relatively small government agency, with an administrative budget of $27 million and 132 staff, but its role as the gatekeeper for medicines in New Zealand gives it outsized power.
Pharmac's decision-makers sit on clinical committees that assess medical efficacy and recommend medicines for use, and another committee that ranks those recommendations for funding. The Government gives it a budget and it funds the best-value medicines first, working through its ranked list until the money runs out.
Last year, Pharmac spent just over $1 billion on medicines.
Most New Zealanders — 3.74 million people — get a subsidised prescription of some kind.
To those who support the model, Pharmac has been a technocratic marvel, providing Kiwis access to a wide range of medicines, vaccines and treatments at a prudent cost. It has meant New Zealand has largely avoided the exploding medicine prices paid by consumers and governments in other countries.
Last year, a report by the New Zealand Initiative, a pro-free market think tank, gave a positive assessment of the model.
"Pharmac, on the evidence you can get, has done an excellent job," said the report's author, economist Bryce Wilkinson. "The comparison with Australia illustrates that."
New Zealand spends less per capita on medicines than Australia, despite filling more prescriptions, according to Wilkinson's paper, The Right Prescription? Another report, by the US-based Rand Corporation, on the price of insulin, a lifesaving medicine for people with diabetes, found Pharmac was paying 92 per cent less than American consumers.
The cost savings aren't easy to achieve. "Only by being hard-headed in commercial negotiations over price, year in and year out, can Pharmac make room in a tight budget for funding costly new medicines," Wilkinson said.
Dr Graeme Jarvis, head of Medicines New Zealand, a drug industry group, said the relationships between Pharmac and pharmaceutical companies are sometimes "more transactional than it needs to be'' and this causes "a sense of frustration" in the industry.
A former drug industry executive told the Herald drug companies find Pharmac difficult to negotiate with. To the ministers who oversee the agency, that's a sign Pharmac is doing its job effectively. "Pharmac is not the best friend to any pharmaceutical company," said the health minister, Andrew Little. "Their job is to drive hard to get the best price."
The funding of hepatitis C drugs shows how the system can work.
A decade ago, the only effective treatment for the virus was a long course of Interferon, which was largely ineffective and debilitating. It required extensive periods of hospital-monitored infusion.
"Overall cure rates were only about 25 per cent for people with hepatitis C, and they were poorly tolerated," said Edward Gane, a hepatologist at Auckland District Health Board.
Warwick said she tried Interferon, but "it didn't work. That was unfortunate, and it was an ordeal. It had a huge impact on my family and relationships and I think it would probably be on par with going through chemotherapy for six months."
Fortunately, science was progressing. In 2009, a small drug company, Pharmasset, began trials of a drug that promised to be more effective, with fewer side effects, and which could be taken at home in pill form. Trials of the drug took place in Auckland and Christchurch, under Gane's supervision.
All 40 patients involved in the trial were cured of hepatitis C. "It was dramatic," Gane said. "People realised it was going to be a paradigm shift."
Then the reality of the pharmaceutical business took over. The small company for which Gane had been running trials was acquired by Gilead, a pharmaceutical giant, which commercialised the breakthrough. In 2013, it rolled out Harvoni (which contains sofosbuvir) at a cost of $120,000 for each course of treatment, a price that raised eyebrows in the health sector and dashed hopes of people like Warwick whose lives could have been transformed by the drug.
At that price, it would have cost $6 billion to cure every hepatitis C sufferer in the country — six times New Zealand's annual medicines budget.
Pharmac and Gilead spent years negotiating behind closed doors. Documents obtained by the Herald revealed these discussions were tough. Gilead, according to Pharmac's internal notes, "generally bases its pricing on the gross national income of the country it is supplying to", so New Zealand didn't have much leverage.
But then the emergence of another treatment — Viekera Pak, produced by AbbVie — changed the dynamic. Viekera Pak would only be effective for 57 per cent of New Zealanders with a specific hepatitis C genotype, but its arrival on the market broke the deadlock in talks with Gilead. Pharmac funded both drugs in 2016.
Initially, Harvoni was provided at a list price of $67,960 for a course of treatment only to those who were near death. Another step forward came in 2019 when AbbVie introduced Maviret, which provided a 95 per cent cure across all hepatitis C genotypes at a cost of $24,750 per treatment.
Pharmac renegotiated, sliced more off the price, and in 2019 Maviret was funded for anyone with a diagnosis. To date, more than 8000 people have received one of the cures. Gane said demand for liver transplants has collapsed and the annual number of deaths from hepatitis C, which was about 170, is diminishing. He hopes by the end of the decade it will be zero.
Gane can now contemplate what was once unimaginable: the end of his field of study. "Don't worry, I'm not despondent," he said. "I've seen too many people die preventable deaths."
But the Pharmac model also has its detractors. Critics say that the process for approving drugs can be drawn-out and secretive. And the agency's hard-headed pursuit of cost savings can lead to disappointment for patients and their families whose lives depend on getting expensive treatments.
People like Stacey Lory in Napier.
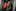
Ten years ago, Lory and her partner became parents for the first time: A son they named Liam. But Lory, who trained in early childhood education, knew early on something was wrong.
"Putting him on his tummy, he never held his own head up," she said. "He started rolling, then he stopped."
At 18 months, Liam was given a provisional diagnosis of spinal muscular atrophy. The disorder, which is found in about four babies born in New Zealand every year, causes malfunctions in the spine, weakening muscles needed for walking, eating and breathing. In its most severe form, life expectancy is measured in months, while those with less crippling varieties face a life of decline.
With no treatment options, Lory said the medical advice they received at the time of Liam's diagnosis was: "Go home and love him for as long as you can."
Lory gave up her career to become a fulltime carer. Annual leave was all spent at Auckland's Starship children's hospital. Their dreams of owning a home turned instead into battles to find wheelchair-accessible rental accommodation.
Then came a glimmer of hope, when researchers at the University of Massachusetts made a scientific breakthrough. In 2016, the world's first treatment for SMA came to market: Spinraza, manufactured by Biogen, a large American biotechnology company. Lory assumed it would soon be available here to people like Liam.
"It never crossed our mind that New Zealand wouldn't fund it," she said.
But the effective new drug came at an exorbitant cost. Spinraza's list price is $177,500 per 5ml vial. Treatment requires six vials administered by spinal infusion in the first year to establish a loading dose, followed by another three vials annually. It is said to be one of the most expensive pharmaceutical treatments ever made available for sale.
The price was a sticking point around the world. Denmark declined to fund Spinraza beyond the most severe category of infants, and the United Kingdom refused funding initially, before striking a deal for wider access in 2019. Australia allocated A$240m for the drug in its 2019 budget after the prime minister's wife, Jenny Morrison, became an ambassador for SMA.
In New Zealand, Pharmac's therapeutic committee acknowledged the evidence for Spinraza's efficacy in 2018 and recommended its use, but it has yet to be funded because of concerns about whether it is cost-effective at its price. Pharmac and Biogen have been engaged in grinding negotiations for two years.
While these talks take place behind closed doors, the likes of Lory have been campaigning publicly for the drug to be funded. So far, their calls have gone unheeded. Biogen recently submitted its eighth offer to sell the drug, after the previous seven were rejected by Pharmac as unaffordable.
Now 10, Liam has weakened to the point he needs a wheelchair to get around. He has a feeding tube installed and is hooked up to three machines overnight to assist with his breathing. The family's morning routine has become a blur of changing nappies, feeding tubes and helping Liam get dressed.
"I've been told so many times: 'You're strong. How do you do it?'" Lory said. "But I don't have a choice. He's my child. I will do anything for my child, just like any other parent would."
She just wants the headbutting between Pharmac and Biogen to be resolved so kids can get the treatment. "They can argue for as long as they want," she said, "but there are still lives at risk. There are children fighting for their life every single day."
Patient advocates say the cost of providing Spinraza in New Zealand would be about $6m annually, an estimate that has been widely reported in the media. But the likely cost is much higher.
Pharmac holds such information closely, but internal documents obtained by the Herald suggest the cost over five years would be around $120m, with a bill for the drug in the first year of $33.7m.
To put that another way, the annual cost of Spinraza, which would help save the lives of four babies and arrest the decline of about 50 other SMA sufferers for a single year, would be roughly equivalent to that of curing 1000 cases of hepatitis C with Maviret.
Spinraza has no real competition. Its patent is not due to expire until 2030. A one-off gene therapy cure for SMA called Zolgensma was recently approved for use in the US by the Food and Drug Administration, but it comes with a list price of $3m.
The cost of Spinraza has prompted criticism overseas. The Institute for Clinical and Economic Review, an American non-profit body that analyses medical treatments, said in an assessment it needed its price to be discounted by 85 per cent to be considered cost-effective.
"Spinraza has proven to be a highly effective drug for a horrible disease," said ICER's David Whitrap in its analysis. "But Spinraza's actual price surpasses even this high benchmark for what a fair US price would be."
Its maker, Biogen, declined to make a spokesperson available to answer questions from the Herald. In a written statement, the company said the price of Spinraza "was determined through a rigorous and thoughtful process" which balances value to patients and society with its need to sustain its business model.
The drug company added that while it negotiates with Pharmac it is also providing the medicine free to nine New Zealand infants with the most serious form of SMA.
The argument about Spinraza illustrates the wider reality that Pharmac faces. Advances in medical science have led to new treatments that promise dramatic improvements for people with rare conditions, but suppliers are demanding premium prices. And New Zealand, as a small market, has limited clout in negotiations with those pharmaceutical giants.
"The reality is that if their medicines weren't so expensive, we'd be able to fund them," Sarah Fitt, Pharmac's chief executive, told the Herald.
Jarvis, the industry's local spokesperson, defended the pharmaceutical companies. "I've invested on average $2 billion and after the patent expires, generally six years if I'm lucky, suddenly people can make a copy of the medicine," Jarvis said. "You've got a limited time to get back money you've invested."
But independent industry analysts are sympathetic to Pharmac's position. Maxim Jacobs, an analyst at Edison Investment Research in New York who has covered the industry for two decades, said drug pricing has become untethered from market forces as costs are borne collectively through either private insurance (US) or public health (NZ) schemes.
"There is no free market," Jacobs said. "The end user is not even paying a fraction of the actual cost of the medicine. It's the insurance companies, whether public or private," he said.
"What the pharmaceutical companies did was very logical for their business. They discovered how high they could go with prices and kept pushing the barrier. And they realised there's almost no limit."
Wilkinson, of the New Zealand Initiative, said: "Pharmac has got to look at how to spend its money. If you've got a drug with which you can save 10 lives, whereas for the same money you could save one, what do you choose?"
"That's the reality," he said. "You can't save everybody."
Even Pharmac's critics admit it is in a difficult position.
Fitt said decisions it makes on which medicines to fund could only be frictionless if it had an unlimited budget.
"No matter how much money we have, there'll always be the next thing that comes along," she said. "You could put a lot more money into Pharmac, and there'd still be stuff on the list we couldn't afford."
But can the model survive?
The panel conducting the review — which includes the former Consumer NZ boss Sue Chetwin and former senior Beehive mandarin Heather Simpson — is expected to present an interim report next month. The review was tasked with addressing criticisms from patients of Pharmac's transparency, complaints by drug-makers about its slow decision making, and whether the agency's core objective should be rethought.
Critics hoping for wholesale reform are likely to be disappointed. The review will not examine Pharmac's decision-making over specific medicines and excluded the fundamental question about the level of resourcing. Its medicines budget, which is determined in the annual budget process alongside all other health spending, is off the table.
Shane Reti, the Opposition health spokesperson, said the review is "toothless and a whitewash" and questions the lack of input from pharmaceutical companies that regularly grapple with Pharmac.
"I want to acknowledge that what we do well is getting a good price for a limited range of preparations," Reti said, "but we need to decide whether that's enough."
Jarvis, of Medicines NZ, also said he is disappointed with the limited terms of reference and called for an audit of how the country is performing on speed and provision compared to its bigger peers.
But Little, the health minister, defended the review's limited framing. He told the Herald he wanted it to address specific complaints about the timeliness of decision-making and transparency, and from sufferers of rare diseases like SMA whose treatments do not fit into the Pharmac's model. But he downplayed the prospect of significant change.
"On balance, we have a pretty good system," Little said. "It's a good model that has served us well for 30 years."

